On 14 October 2024, the Council of the European Union has formally adopted the agreement on the European Disability Card, which aims to enhance mobility for people with disabilities across the EU. This is a positive development for Europeans living with a rare disease, many of whom are affected by a related disability.
The new rules will establish an EU-wide disability card to ensure that persons with disabilities have equal access to preferential conditions, such as reduced or zero entry fees, priority access, and access to reserved parking. As outlined in the adopted text, individuals participating in mobility programmes such as Erasmus+ and third-country nationals.
The European Disability Card will be issued in physical format and, when available, in digital format, and will be issued and renewed free of charge. Depending on the country, costs could be charged for loss and damage to the card.
Next steps
Member States are to implement the directive by 5 June 2028.
Read more here
Formally inaugurated on April 24, 2024, the Critical Medicines Alliance (CMA) was officially launched the next day in Brussels. The EHC, represented by Daria Camilli, Communications and Education Manager, participated in the closed-door launch together with the representatives of other members of the CMA as well as the European authorities.
The full member list can be found here.
Background
This Critical Medicines Alliance is a new consultative mechanism bringing together national authorities, industry, civil society, the Commission, and EU agencies to identify the best measures to address and avoid shortages of critical medicines. It complements regulatory measures in the proposed EU pharmaceutical reform and is at the centre of the new strategic and coordinated industrial approach to enhance the security of supply of the most critical medicines.
The work of the Alliance will focus on critical medicines that face the greatest vulnerabilities, based on the ongoing Commission vulnerability analysis for a sub-set of substances listed on the Union list of critical medicines, first published by the European Medicines Agency (EMA) in December 2023.
More about CMA here.
Next steps
The Alliance will use the outcome of the pilot assessment of critical medicines
as a point of reference
➔ As of May, members of the Alliance will collaborate in thematic Working Groups
➔ Throughout Q4 2024, the Working Groups will make recommendations
based on the identified vulnerabilities
➔ By the end of 2024, the Steering Board will come forward with a Strategic
Plan, which the Alliance Forum will consider and endorse
➔ This plan will establish a multi-annual set of actions aimed at guiding the
work of EU decision-makers
➔ The initial mandate of the Alliance is of 5 years
Further reading
More about the CMA on the EC website here.
Union list of critical medicines here.
On 20 February 2024, the Management Board of ECDC nominated Dr Pamela Rendi-Wagner to be the next Director of ECDC for a five-year period (2024-2029).

“I am delighted to announce that Dr Pamela Rendi-Wagner has been nominated to be the next ECDC Director by ECDC’s Management Board. The Board is convinced that her experience, vision and plans will serve the Centre well for the next five years. We look forward to her upcoming hearing in the European Parliament in March,” said Dr Anni Virolainen-Julkunen, Chair of the ECDC Management Board. “On behalf of the Management Board, I would also like to express our gratitude and appreciation to Dr Andrea Ammon for the excellent work she has done as ECDC Director. We wish Andrea the best as she retires in June 2024”.
Dr Pamela Rendi-Wagner spoke to ECDC staff after the vote and said “I am deeply honoured and grateful to the Management Board for entrusting me with the role of Director of ECDC. I look forward to taking up the position following the hearing at European Parliament’s Committee on Environment, Public Health and Food Safety”.
Each Management Board member had one vote, and the candidate required support from two-thirds of all voting members. The Board members cast their vote by secret ballot. The Management Board of ECDC is composed of one member designated by each Member State, two members designated by the European Parliament, and three members representing the European Commission, all with a right to vote.
Before her appointment, Dr Pamela Rendi-Wagner will be invited to make a statement before the European Parliament and to answer the questions of its Members.
Read the biography of Pamela Rendi-Wagner
Source: https://www.ecdc.europa.eu/en/news-events/new-director-ecdc-2024
MEPs adopted their recommendations on prioritising mental health, as an integral part of a person’s health, in EU and national policies.
The report, prepared by the Subcommittee on Public Health, was adopted with 59 votes in favour, six against and four abstentions. MEPs call on the Commission to draw up a long-term, comprehensive and integrated EU Mental Health Strategy, building on the recent communication, and on national governments to develop corresponding national strategies with clear timelines, adequate budgets, concrete targets and indicators to monitor progress.
Promoting mental health for all, with a focus on vulnerable groups in society
MEPs highlight that mental health and well-being are shaped by a combination of socio-economic, environmental, biological and genetic factors and that any person at any point in their life can become more susceptible to poorer mental health. A “mental-health-in-all-policies approach” is needed to prevent, address and mitigate the impact of mental health conditions, they add. The report calls on member states to prioritise and improve access to mental health services for vulnerable groups, such as children, adolescents, young adults, LGBTQIA+ persons, patients with chronic conditions and disabilities, elderly people, migrants and ethnic minorities.
Tackling discrimination, stigma and social exclusion
With mental health still stigmatised and taboo, MEPs underline that there is an urgent need to develop and implement information campaigns, raise awareness and promote open discussions of mental health conditions. The call on the Commission and EU governments to promote initiatives to combat stigma, exclusion and discrimination of people with mental health conditions, with the involvement of communities, public figures, politicians, public institutions, governments and people with lived experience.
Improving accessibility of mental health services
MEPs insist that all EU citizens must have access to the full range of quality mental health services, without financial and administrative hardship. They highlight the need to ensure further investment in public health and to address mental health workforce shortages and appropriate training.
Other key recommendations include:
- ensuring a safer and healthier digital space, to prevent online hate and cyberbullying;
- tackling gender inequalities and violence against women;
- legislative measures on the management of psychosocial risks and well-being at work;
- collecting and monitoring mental health data, as well as suicide-related data, and mapping the availability of mental health services across the EU;
- ensuring sufficient funding in future financial programs such as EU4Health and Horizon Europe, as well as a Mission on Mental Health;
- designating next year as the European Year of Mental Health, following proposal 9.1 of the Conference on the Future of Europe.
Quote
Rapporteur Sara Cerdas (S&D, PT) said: “Health policy often neglects to address mental health, which is worrisome considering the steady increase in mental health conditions. Mental health is influenced by a number of determinants, including socioeconomic factors, and is interlinked with physical health. With this first Parliament report on the matter, we urge for further EU action to address these factors, investing in prevention, support and adequate treatment for people suffering from mental health conditions and reinforcing the resilience of mental health for all.”
Background
On 7 June 2023, the Commission adopted the Communication on a comprehensive approach to mental health. It introduces 20 flagship initiatives and a holistic approach to mental health, based on adequate and effective prevention, access to high quality and affordable mental healthcare and treatment, and reintegration into society after recovery.
Abstract
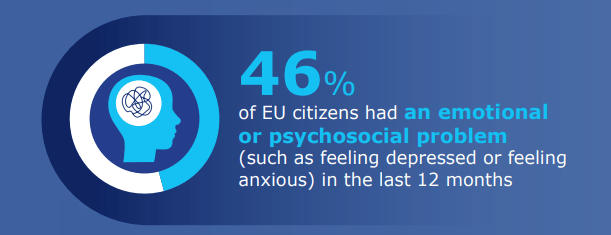
89% of respondents consider that mental health promotion is as important as physical health promotion. At the same time, less than half of respondents agree that people with mental health issues receive the same level of care as those with a physical condition. 46% of respondents have experienced an emotional or psychosocial problem, such as feeling depressed or anxious, in the past twelve months. 54% of respondents with a mental health issue have not received help from a professional. Most respondents replied that recent world events have influenced their mental health ‘somewhat’ (44%) or ‘to a great extent’ (18%).
Key findings
Mental health and recent world events
62% of EU citizens think that recent world events (the COVID-19 pandemic, the Russian aggression against Ukraine, the climate crisis, unemployment, and the food and energy costs “somewhat” or “greatly” affected their mental health.
Factors contributing to good mental health

The most important factors to achieve good mental health are living conditions (60%), followed by financial security (53%), physical activity and social contact (both 41%).
Source: https://europa.eu/eurobarometer/surveys/detail/3032
Over the past three years, EMA and the EUnetHTA 21 (European Network for Health Technology Assessment) consortium have delivered a number of milestones to prepare the EU for the entry into application of the Regulation on Health Technology Assessment. EUnetHTA 21 ceases to operate on 16 September 2023, but preparations will continue for the implementation of the Regulation, under the direction of the HTA Coordination Group.
“EMA’s collaboration with EUnetHTA began in 2010 as a project to test out whether early engagement between regulators and HTA bodies could bring tangible benefits for patient access to medicines,” said Michael Berntgen, Head of Scientific Evidence Generation Department at EMA. “Together, we were able to help medicine developers enhance clinical research and become more efficient in generating the evidence relevant for both regulatory authorities and HTA bodies.”
“Although the EMA-EUnetHTA cooperation is drawing to a close, the road does not end here,” said Niklas Hedberg, Chair of the EUnetHTA 21 Consortium Executive Board. “We are very proud to see that our cooperation has now been enshrined into European law. The experience gained provided essential technical input to shape the new legislation. In fact, we continue our joint work and will do this on a new footing, with more participants, additional responsibilities and high expectations from stakeholders.”
Reviewing achievements over the years at their concluding meeting on 14 September 2023 in Amsterdam, EMA and EUnetHTA 21 highlighted a number of initiatives:
- Completion of seven parallel joint scientific consultations (JSC) for medicines under the EUnetHTA 21 consortium contract. This joint work is intended to improve the generation of robust evidence that meets the needs of regulators and HTA bodies;
- Discussion on evidence needs for advanced therapy medicinal products in oncology, addressing mutual challenges such as indirect comparison and addressing evidence gaps through post-licensing evidence generation.
- Organisation of trainings for patients and healthcare professionals to facilitate their participation as experts in regulatory and HTA processes, alongside collaborative work on methodologies for engagement of patients and healthcare professionals in assessments;
- Recommendations to optimise the assessment reports of EMA’s Committee for Human Medicines (CHMP) for each medicine in order to systematically document key elements of the assessment such as the eligible patient population, choice of comparator and endpoints, as well as relevance of subgroup data.
More information on the achievements is available in a Report on the implementation of the EMA-EUnetHTA 21 work plan 2021 – 2023 .
The new HTA regulation
The Regulation on Health Technology Assessment (EU) 2021/228 which entered into force in January 2022 and applies as of January 2025, will govern the European cooperation between medicine regulators and HTA bodies. Under the new framework, EMA and HTA bodies will collaborate in the context of joint clinical assessments, joint scientific consultations, and the identification of emerging health technologies.
While aiming to improve the availability of innovative medicines and certain medical devices for patients in the EU, it will also ensure efficient use of resources and enhance the quality of health technology assessment in the EU by ensuring the sustainability of European cooperation. The establishment of the Member State Coordination Group on Health Technology Assessment, as provided by the regulation, and of a stakeholder network, will give a transparent and inclusive framework to facilitate continued collaboration between partners and reduce duplication of efforts for national HTA authorities and industry.
Transition period to January 2025
EMA and HTA organisations have established a new framework for Parallel EMA/HTA Scientific Advice for the period September 2023 until January 2025, when the HTA Regulation applies. During this transition period, developers can request the involvement of HTA bodies when applying for EMA scientific advice. The outcome of the procedure will be a scientific advice letter from EMA and individual written recommendations from participating HTA bodies. The selection criteria are available in the Guidance on Parallel EMA/HTA body (HTAb) Scientific Advice for the Interim Period
Preparations are also continuing at EMA to pave the way for the implementation of the Regulation. The Agency has identified a number of priorities and opportunities for the next 15 months. These include defining a single evidence plan to facilitate development programmes, harmonising views on the strength of the evidence, and involving patients, clinical experts and other relevant experts in decision-making.
Notes
- The EUnetHTA 21 consortium had been awarded the service contract for the provision of joint health technology assessment (HTA) work supporting the continuation of EU cooperation on HTA by the European Health and Digital Executive Agency (HaDEA).
